Por: Rafael Roca, MESc/MBA 2016*
Claudia Zúñiga Carrillo**
Abstract
Floristic inventories of tropical forest after 20 years of artisanal gold mining practices in Nyungwe National Park, Rwanda, were undertaken to understand the effect of this mining on forest composition and diversity. All selected sites had suffered severe mining impacts by 1993. Three plots of 0.05 ha were surveyed in each of four locations (Karamba, Rugazi, Akabaguri, and Mugote), and all trees >10 cm DBH were censused. We found a total of 215 individual trees of 23 species and 18 families. The most common and dominant species was Hagenia abyssinica (Rosaceae), while the most frequent was Dichaetanthera corymbosa (Melastomataceae). In order of importance, Dichaetanthera corymbosa, Anthocleista grandiflora, Maesa lanceolata, Chionanthus africanus and Afrocrania volkensii were the indicator species in Karamba; Dichaetanthera corymbosa, Syzygium guineense, Hagenia abyssinica, and Anthocleista grandiflora were the indicator species in Rugazi; and Hagenia abyssinica was the indicator species in Akabaguri and the sole species found in Mugote (fire recovery site). The mixing ratio of the mining recovery sites range from 0.19 to 0.21, suggesting the regeneration of heterogeneous ecosystems.
Introduction
Mining is a well-known destructive practice within many forests around the world (Dudka & Adriano 1995). Trees are cleared, topsoils and organic litter removed, and water streams are modified (Cooke & Johnson 2002). Such disturbances have an impact on nutrient cycling processes and forest regrowth (Congdon et al. 1993). Legal mines are obliged to comply with environmental commitments, and often set up a plan to achieve a desired and approved state of restoration. The ultimate long-term objective is that mined sites return to the state of premined forest, with the presence of all plant species at the same frequency and density as before (Koch 2007). However, attempted restoration of mined sites in Brazil and Australia have shown contrary results in returning these sites’ ecosystems back to a pre-mining state. Some studies indicate successful forest regeneration already advanced through secondary succession (Rodrigues et. al 2004), while others show that rehabilitated sites are not becoming more similar to the unmined forest over time (Norman et al. 2006). Understanding the factors that aid or inhibit successful restoration is a key question in determining the long-term effects of mining and other destructive impacts on forests. In 2014, Rwanda was considered the 19th most densely populated country in the world, averaging 460 inhabitants per km2 (World Bank, 2016). This is no exception in Nyungwe National Park, where family farms located within or near the National Park’s buffer zone average less than 1 hectare in size, and over 90% of the population engage in subsistence farming (Mazosera et al. 2006). To supplement this livelihood, local communities historically have used the forest to generate additional income through gold mining, wood cutting, hunting, and honey collection. Even though Nyungwe has had some form of protection since it was established as a forest reserve in 1933, accumulated deforestation between 1958 and 1979 was estimated at 15% (Weber 1989). Further, in 1993 an estimated 2,528 artisanal miners were living and working within 16,000 ha of Nyungwe (Fimbel & Kristensen, 1994). To mediate this threat to the National Park’s continued conservation, Fimbel & Kristensen (1994) proposed establishing policies that would permit miners to continue their operations within already impacted areas but limit their spread into highly biodiverse zones. Since then, strong enforcement after the genocide, the establishment of Nyungwe as a National Park in 2004, and constant efforts by conservation organizations (such as the Wildlife Conservation Society and the Rwanda Development Board) have led to implement controls that support the complete ban on mining, hunting, and extraction of other forest products within the Park. Enforcement registers indicate that from 2006 to 2010 there were at least 227 poachers arrested (Mulindahabi et al. 2011). Tourism has co-evolved with conservation, alleviating some of the impact generated by illegal activities within Nyungwe. From 2001 to 2014, the number of tourist visits increased from 5,965 to 67,871, an average of 18% per year (Rudasingwa 2014). However, despite the associated increase in income from tourists (in 2014, Nyungwe’s tourism revenues increased by 37%), this revenue source amounts to only 2% of the estimated $16.8 million of total revenue generated by Rwanda’s National Parks system. Most revenue comes from gorilla trekking in Volcanoes National Park, accounting for 94% in 2014. Revenue streams in Nyungwe include trail walks (27%), primates visits (29%), and canopy walks (38%) (Rudasingwa 2014). A system of revenue sharing among all Parks has been set up, where 5% of all revenues are directed to communityprojects bordering the parks. These revenues are apportioned 40% to communities located near Volcanoes National Park and 30% each to Akagera and Nyungwe National Parks. Despite this effort, illegal activities still occur within Nyungwe National Park and nearby communities still rely on forest resources or extractive mining to sustain their livelihoods.
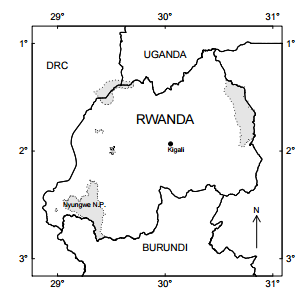 Fig. 1. Map of Rwanda, showing Nyungwe National Park.
Fig. 1. Map of Rwanda, showing Nyungwe National Park.
Artisanal gold mining continues, but the limited area generates poor returns and it is viable only for subsistence purposes. Mining began in 1935, with records reporting as many as 12,000 miners between 1972 and 1985 (Fimbel & Kristensen 1994). Miners use alluvial techniques, deforesting sites close to watersheds, burning them to later extract the sediments from the soil. Sifting is common to separate the gold and is considered a safer and less environmentally harmful technique than using mercury, a practice still common in the Peruvian Amazon (Ashe 2012). However, negative correlations between signs of gold mining and ungulate populations (Plumptre et al. 2002) suggest that miner’s intensive hunting practices have been highly detrimental for the forest (Weber 1989). Atpresent, illegal miners tend to work fast and move to new remote areas, instead of setting up camps in previously established sites. Mined forest in Nyungwe, Rwanda, has been left undisturbed for the past 20 years, and no restoration activities other than non-human natural interactions have taken place. To date, no study has documented the ecological recovery of mined areas within Nyungwe and there is little understanding of the environmental impacts of this activity and how the forest responds to such stress. This paper examines the regeneration of areas mined in 1994 to determine the speed and trajectory of forest recovery and asks whether mined forest is likely to return to its previous state.
Methods
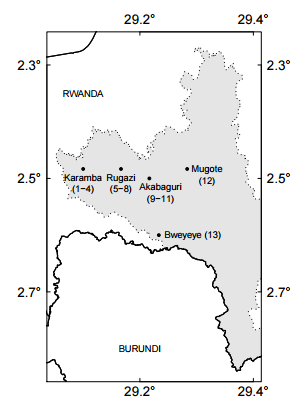
Study Site Nyungwe National Park is Rwanda’s largest standing forest at 970 km2 . It is located in the southwest (latitude 2°15’ and 2°55’S, longitude 29°00’ and 29°30’E) and extends along mountainous areas that range from 1600 to 2950 meters above sea level (Weber 1989, Masozera et al. 2006, Fig. 1). Data from 1988–1993 suggests average annual rainfall of 1,744 mm, fluctuating considerably between July–August (dry season) and other seasons. Temperatures are typical for a tropical region, with daily variation greater than annual variation (average minimum temperature = 10.9°C, and average maximum temperature = 19.6°C; Sun et al. 1996). Nyungwe National Park is highly biodiverse, with 86 recorded species of mammals, 280 birds, 43 reptiles, 33 amphibians, 1105 plants (ferns, herbs, climbers and shrubs) and 230 trees; both represent 137 out of 551 endemic species found in the Albertine Rift (Plumptre et al. 2007). Thirteen species of primates have been recorded within Nyungwe, including chimpanzees, owl-faced guenons and Angolan black and white colobus monkeys in groups of 300+ individuals (Fimbel et al. 2001, Plumptre et al. 2002) The selection of study sites was based on sites classified as suffering severe mining impacts by 1993, and accessibility to the areas. Information on the sites was based on the mining census elaborated by Fimbel and Kristensen (1994). Specific locations (Appendix A) within the sites were randomly determined once the affected areas were clearly identified. Local park rangers validated the fact that these areas have not been mined in approximately 20 years.
Floristic Inventory
The vegetation and floristics of the previously mined forest sites were assessed with a series of 20 x 25 m (0.05 ha) tree plots within which all trees >10 cm diameter at breast height (DBH, 1.3m) were recorded and identified (Synnot 1979). Each tree was measured for DBH, height (electronic altimeter), and projected canopy area. All trees were identified to species. Plots were established at three sites: Karamba (4 plots, 0.2 ha total), Rugazi (4 plots, 0.2 ha total), and Akabaguri (3 plots, 0.15 ha total) (Appendix A). Plots were randomly located within each mined site if the shape allowed. In addition, we measured one plot (0.05 ha) near Mugote, an area burned by an uncontrolled fire in the 1990s, with the purpose of comparing the ecological recovery of mining and fire sites. Finally, a new recently found illegal mining site was visited near Bweyeye, found by local park rangers around May 15th 2015. They claimed that miners must have been there about two weeks before. The objective of this visit was to visualize the direct immediate effects of mining on the land, with the purpose of having a better understanding of the areas studied almost 20 years after. Data analysis For each plot, we calculated the Importance Value Index (IVI, Equation 1) for each species. This statistic is a summation index that encompasses relative density plus relative frequency plus relative dominance for each species, with a value of 300 as the total for all species within a site (Curtis and McIn-tosh 1951). This index allows the comparison of the ecological weight of each species within its ecosystem. Similar IVI’s for indicator species in different plots suggest similar forest composition, structure, and dynamics (Lamprecht 1990). Indicator species are those that form the top 150 IVI. IV I = RA + RF + RD (1) where RA = relative abundance, RF = relative frequency, and RD = relative dominance. For each species, we calculated relative abundance: RA = (ni/N) ∗ 100 (2) where ni = number of trees of species i and N = total number of trees of all species; relative frequency: RF = (Fi/Ft) ∗ 100 (3) where Fi = absolute frequency of species i and Ft = the total number of absolute frequencies; and relative dominance: RD = (Gi/Gt) ∗ 100 (4) where Gi = total basal area of all the trees of species i and Gt = total basal area of all the trees of all species. Finally, we calculated the mixing ratio, an indicator that measures the degree of homogeneity or heterogeneity within the forest (Lamprecht 1990, CM): CM = S/N (5) where S = the total number of species within the sample and N = the total number of trees within the sample.
Fig. 2. The location of sites in Nyungwe National Park, Rwanda.
Results
Within the twelve 0.05 ha plots, we found a total of 215 individual trees >10 cm DBH in 23 species and 18 families. Density in each plot ranged from 7 to 24 trees (mean = 18, SD = 6). DBH ranged from 10 to 65 cm (mean over all trees = 17.1, SD = 8.5; mean per plot = 17.5 cm, SD = 3.4). The species richness of each site varied considerably. Karamba was the richest with 16 species, followed by Rugazi (14 species), Akabaguri (9) and Mugote (1). Karamba and Rugazi had the same tree density (75) over the same number of plots. The most common species over all individuals on the three mining sites was Hagenia abyssinica with 42 trees, despite its absence in Karamba. The next most common was Dichaetanthera corymbosa with 32 trees. Eighteen species had less than 10 individuals and six species were present as single individuals
Table 1. Indicator species for each site (those summing to at least an IVI of 150. Full details of each site are given in the Appendix
The species frequency was best represented by Dichaetanthera corymbosa and Anthocleista grandiflora in Karamba, Dichaetanthera corymbosa, Macaranga kilimandscharica and Hagenia abyssinica in Rugazi, and Polyscias fulva and Hagenia abyssinica in Akabaguri. The most frequent species on these sites was Dichaetanthera corymbosa, present on 82% of the mined plots. The next most frequent was Polyscias fulva with 73% presence. Seven species were found in only one plot. Overall dominance was greatest for Hagenia abyssinica with 29% of relative dominance among the mining plots. The next most dominant species was Dichaetanthera corymbosa with 16%. The species dominance was best represented by Dichaetanthera corymbosa in Karamba, Syzygium guineense and Dichaetanthera corymbosa in Rugazi, and Hagenia abyssinica in Akabaguri. In order of importance, Dichaetanthera corymbosa, Anthocleista grandiflora, Maesa lanceolata, Chionanthus africanus and Afrocrania volkensii were the indicator species in Karamba, Dichaetanthera corymbosa, Syzygium guineense, Hagenia abyssinica and Anthocleista grandiflora were the indicator species in Rugazi, and Hagenia abyssinica is the indicator species in Akabaguri (Table 1). All the sites are fairly similar when discussing the mixing ratio, ranging from 0.19 to 0.21 (Table 2). Discussion In eleven 0.05 ha tree plots in previously mined forest sites in tropical forest in Nyungwe National Park, Rwanda, we documented 195 trees >10 cm DBH in 23 species. Two species were dominant in terms of abundance (Hagenia abyssinica and Dichaetanthera corymbosa), two were dominant in terms of frequency (Dichaetanthera corymbosa and Polyscias fulva), and two in terms of basal area (Hagenia abyssinica and Dichaetanthera corymbosa). These data presents some of the first information on forest regeneration following gold-mining in tropical Africa. Species diversity of 20-year old mined forest There are over 100 species of large trees (>30 cm DBH) in Nyungwe National Park (Plumptre et al. 2002). However, more than half of the individual trees come from only five species: Syzygium guineense, Macaranga kilimandscharica, Carapa grandiflora, Strombosia scheffleri, and Hagenia abyssinica (Fashing et al. 2007). Of these, only Strombosia scheffleri was absent from our census. Out of the top 20 species of large trees, 10 were also registered in this study (Plumptre et al. 2002). Tropical forests tend to have a high number of species per area, usually showing a mixing ratio of at least 0.2 (Malleux 1987). This claim suggests that these sites are recovering in a way that promotes a heterogeneous ecosystem. Table 2. Mixing ratio (number of species / number of individuals) of each site.
Important and indicator species in mined forest Indicator species (those in the top 150 IVI) were similar across the sites, with Dichaetanthera corymbosa and Anthocleista grandiflora present at both Karamba and Rugazi. Both sites had similar species richness (16 and 14 species) and stem density (75 trees >10 cm DBH). In contrast, Akabaguri had one indicator species, Hagenia abyssinica. This species was also an indicator species for Rugazi and was the sole species found within the Mugote plot, in the fire-recovered area close to the Congo Nile Trail (Appendix B). Other studies have suggested that it is a fire-tolerant pioneer species (Finch and Marchant 2011, Lange et al. 1997, Greenway 1973; White 1983; Lovett et al. 2006). This native species also has the ability to produce leaf litter that decomposes quickly, adding nutrients to disturbed soils (Assefa and Glatzel 2010). Despite these properties, its use as a construction material, firewood, medicine and livestock fodder in other parts of Africa, such as Ethiopia, has led to decreasing populations (Tegegne and Mekonnen 2007; Assefa and Glatzel 2010). Dichaetanthera corymbosa, Anthocleista glandiflora and Maesa lanceolata have been identified respectively in Congo, Equatorial Guinea and Nyungwe as signs of secondary succession after periods of human disturbance in the form of shifting and semi-permanent cultivation, i.e., cacao (Yamada 1999; Zafra Calvo 2008; Graham, Moermond et al. 1995). Conclusion The ecological recovery of these mining sites shows promising results for the future. Natural regeneration is taking place with native species that are able to adapt to extreme conditions after being mined. This is a slow process, but the indicators suggest that diverse pioneer species have already established themselves, and will allow the forest to eventually recover through its natural dynamic process. To understand this better, further long-term studies should be undertaken, including a re-assessment of these sites in the future, and their comparison to young and mature forest within Nyungwe using larger sample areas. If left undisturbed by human activity, these forest sites may well return to forest similar to the natural oldgrowth forest. The objective of this preliminary study has been to pursue an initial understanding of the state of abandoned artisanal gold mining sites in Nyungwe. With these results, showing slow but diverse natural recovery by pioneer species, we point out that strongly positive outcomes have resulted from the conservation efforts of Rwandan authorities and local actors. Further forest succession would be expected with continued law enforcement and development of alternative sources of livelihoods to reduce pressure to the forest. Artisanal gold mining remains a threat, especially in more remote areas. The site observed near Bweyeye (Appendix A) suggested a short-term stay with intense labor, and the possibility that miningand miners move readily between Rwanda and Burundi. Given the nature of subsistence of this activity, it is vital to understand the new sources of revenues of past miners, and promote economic development in a way that makes it more profitable for them to pursue legal alternative activities. This will have a significant contribution to the conservation of Nyungwe National Park. It is therefore imperative to complement the current efforts successfully undertaken through tourism to continue reducing pressure towards the forest.
Acknowledgements
We are extremely grateful to Nzakizwanayo Eraste (WCS), who identified all the trees in our study plots. This study was supported by funding from the Lindsay Fellowship and Jubitz Family Endowment at Yale University. We appreciate WCS logistical support in the form of accommodation, transport and human resources. WCS and Rwanda Development Board made it possible to research the sites with a local team. We would like to extend our most sincere thanks to Michel Masozera, Nicolas Ntare, Felix Mulindahabi, Antoine Mudakikwa, Amy Vedder, Bill Weber, and the local park rangers for their contributions.
References
Ashe, K. 2012. Elevated mercury concentrations in humans of Madre de Dios, Peru. PloS One 7, e33305.
Assefa, B. and Glatzel, G. 2010. Measuring soil fertility under Hagenia abyssinica (Bruce) J.F. Gmel by the Biotest method. International Journal of Agronomy 2010, 845087.
Congdon, R.A. and Herbohn, J.L. 1993. Ecosystem dynamics of disturbed and undisturbed sites in north Queensland wet tropical rain forest. I. Floristic composition, climate and soil chemistry. Journal of Tropical Ecology 9, 349–363.
Cooke, J.A. and Johnson, M.S. 2002. Ecological restoration of land with particular reference to the mining of metals and industrial minerals: A review of theory and practice. Environmental Reviews 10, 41–71.
Curtis, J.T. and McIntosh, R.P. 1951. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 32, 476–496.
Dudka, S., and D. C. Adriano. 1997. Environmental impacts of metal ore mining and processing: A review. Journal of Environmental Quality 26, 590–602
Fashing, P.J., et al. 2007. Activity and ranging patterns of Colobus angolensis ruwenzorii in Nyungwe Forest, Rwanda: Possible costs of large group size. International Journal of Primatology 28, 529–550.
Fimbel, C., et al. 2001. An ecological basis for large group size in Colobus angolensis in the Nyungwe Forest, Rwanda. African Journal of Ecology 39, 83–92.
Fimbel, R. and Kristensen, K. 1994. Gold Mining Activities within the UGZ 4 Management Zone, Nyungwe Forest, Rwanda. Report to WCS. Finch, J. and Marchant, R. 2011. A palaeoecological investigation into the role of fire and human activity in the development of montane grasslands in East Africa. Vegetation History and Archaeobotany 20, 109–124.
Fischer, E., & Killmann, D. 2008. Illustrated Field Guide to the Plants of Nyungwe National Park Rwanda. Department of Geography of The Institute for Integrated Natural Sciences, University of Koblenz-Landau, Campus Koblenz, Rwanda.
Graham, C.H., Moermond, T.C., Kristensen, K.A., & Mvukiyumwami, J. 1995. Seed dispersal effectiveness by two bulbuls on Maesa lanceolata, an African montane forest tree. Biotropica 27, 479–486.
Koch, J.M. 2007. Restoring a jarrah forest understorey vegetation after bauxite mining in Western Australia. Restoration Ecology 15, S26–S39.
Lamprecht, H. 1990. Los Ecosistemas Forestales en los Bosques Tropicales y sus Especies Arbóreas: Posibilidades y métodos para un aprovechamiento sostenido. Cooperación Técnica-Alemania, Eschborn, Germany.
Malleux, J. 1987. Assessment of Woody Biomass Changes in Forest Land. Ad Hoc FAO/ECE/FINNIDA Meeting of Experts on Forest Resource Assessment, Kotka (Finlandia), 26-30 Oct 1987.
Masozera, M.K., Masozera, M.K., Alavalapati, J.R., Jacobson, S.K., & Shrestha, R.K. 2006. Assessing the suitability of community-based management for the Nyungwe Forest Reserve, Rwanda. Forest Policy and Economics 8, 206– 216.
Mulindahabi, F. Aaron N., Rugerinyange L. 2011. 5-Year Ranger-Based Monitoring Comparison Report 2006 to 2010. Report to WCS and Rwanda Development Board.
Norman, M.A., Koch, J.M., Grant, C.D., Morald, T.K. and Ward, S.C. 2006. Vegetation succession after bauxite mining in Western Australia. Restoration Ecology 14, 278–288.
Plumptre, A., Masozera, M., Fashing, P.J., McNeilage, A., Ewango, C., Kaplin, B.A. and Liengola, I. 2002. Biodiversity Surveys of the Nyungwe Forest of southwest Rwanda: Final Report. Wildlife Conservation Society Working Papers19 1-95.
Plumptre, A.J., Davenport, T.R., Behangana, M., Kityo, R., Eilu, G., Ssegawa, P., Ewango, C., Meirte, D., Kahindo, C., Herremans, M. and Peterhans, J.K. 2007. The biodiversity of the Albertine Rift. Biological Conservation 134, 178–194.
Rudasingwa, J. 2014. National Parks in Numbers— Highlights of 2014. Report to Rwanda Development Board.
Rodrigues, R.R. Martins, S.V. and de Barros, L.C. 2004. Tropical rain forest regeneration in an area degraded by mining in Mato Grosso State, Brazil. Forest Ecology and Management 190, 323–333.
Sun, C., Kaplin, B.A., Kristensen, K.A., Munyaligoga, V., Mvukiyumwami, J., Kajondo, K.K. and Moermond, T.C. 1996. Tree phenology in a tropical montane forest in Rwanda. Biotropica 28, 668–681.
Synnott, T.J. 1979. Manual of Permanent Plot Procedures for Tropical Rainforests. Dept. of Forestry, Commonwealth Forestry Institute, University of Oxford.
Tegegne, K.M. 2007. Evaluation of Selected Indigenous and Exotic Tree and Shrub Species for Soil Fertility Improvement and Fodder Production in the Highland Areas of Western Shewa, Ethiopia. Austrian Academy of Sciences. Weber, A.W. 1989. Conservation and Development on the Zaire-Nile Divide: An analysis of value conflicts and convergence in the management of Afromontane forests in Rwanda. PhD Thesis, University of Wisconsin-Madison, Madison, USA.
Yamada, T. 1999. A report on the ethnobotany of the Nyindu in the eastern part of the former Zaire. African Study Monographs 20, 1–72.
Zafra Calvo, N. 2008. Planificación Sistemática de la Conservación en la Isla de Bioko, Guinea Ecuatorial. PhD Thesis, University of Alcalá, Spain. URL: http://fullertl.bol.ucla.edu/CalvoDissertation .pdf
Notas
* Rafael Roca is a forestry engineer, specializing in tropical rain forest conservation and development.
**Claudia Zuñiga is a forestry engineer and specialist in Tropical Silviculture. She is an MSc candidate in Environmental Science at La Molina National Agrarian University, Perú.
See the paper in “TROPICAL RESOURCES”. The Bulletin of the Yale Tropical Resources Institute: https://goo.gl/R90abv